Abstract
Introduction
The twice daily dosing of nilotinib in the absence of food is a considerable burden for patients with chronic myeloid leukemia (CML). In order to simplify the dosing regimen and reduce treatment costs while taking advantage of the food dependent bioavailability of nilotinib, in the present study, we investigated the effects of real-life food consumption on the pharmacokinetics (PK) of nilotinib in chronic phase CML patients.
Methods
Nilotinib 300 mg BID administered under fasting conditions (reference treatment) was compared with nilotinib at a reduced dose of 200 mg BID under fed conditions (test treatment). Food intake was not standardized, in order to increase implementability in general practice. However, to ensure the intended increase in bioavailability, the size and composition of the meal were verified and food intake increased if necessary. Blood samples were collected at two days during each of the two treatments, with sampling time points at 1, 2, 3, 4, 6, 9 and 12 hours after nilotinib intake in the morning and 1, 2, 3, 4 and 12 hours after nilotinib intake in the evening. Plasma concentrations of nilotinib were measured by means of dried blood spot sampling. Geometric means ratio (90% confidence interval [CI]) were determined using a paired samples t-test on log-transformed PK parameters AUC0-12 h, Cmin and Cmax. Bioequivalence was concluded if the 90% CIs were contained within the equivalence limits of 80%-125%. Adverse events were recorded by means of a patient diary and ECG-monitoring. Quality of life was measured using EORTCs QLQ-C30 and QLQ-CML24 questionnaires.
Results
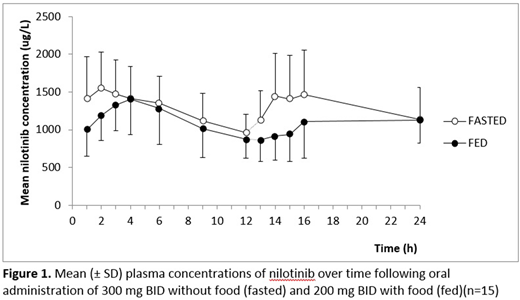
Fifteen patients aged 40-74 years participated. The arithmetic mean concentration-time profiles of the two dosing regimens studied are shown in Figure 1. Nilotinib absorption was somewhat delayed following the administration of nilotinib with food. The geometric mean values (90% CI) for nilotinib AUC0-12 h, Cmin and Cmax following morning intake with food decreased by 11% (81-98%), 12% (84-92%), and 10% (80-102%), respectively. Following evening intake, the geometric mean values for AUC0-12 h and Cmax decreased by 16% (73-97%) and 20% (68-93%), respectively, and increased by 6% (92-122%) for Cmin. For both dosing regimens considerable intra- and interindividual differences in nilotinib PK occurred. No QT-interval prolongations were observed, and for both regimens the frequency of AEs was similar. The EORTC QLQ-CML24 'symptom burden' score was significantly better for the intake of nilotinib with food (p<0.05).
Conclusion
Using nilotinib at a reduced dose of 200 mg BID under fed conditions in patients with chronic phase CML seems feasible and safe. Bioequivalence, however, was not completely conclusive. In spite of somewhat decreased PK parameters (10-12%), bioequivalence was established in terms of AUC0-12 h, Cmin and Cmax following morning intake. Following evening intake, bioequivalence was established in terms of Cmin, but not of AUC0-12 h and Cmax. In addition to monitoring high intra-patient variability, it is advisable to use therapeutic drug monitoring when implementing the intake of a reduced dose of nilotinib with food in clinical practice.
Janssen:Jazz pharmaceuticals: Consultancy; BMS: Research Funding; Novartis: Research Funding; Incyte: Consultancy, Speakers Bureau; Abbvie: Consultancy; Pfizer: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.